- Researchers treated all patients with cisplatin with or without vinorelbine, docetaxel, gemcitabine or pemetrexed.
- Study examined effects of therapy in patients previously treated with bevacizumab.
- All patients had non-squamous non-small cell lung cancer.
- Study was not randomized.
Which adjuvant chemotherapy works best following surgery in NSCLC? It’s hard to say. However, a study that compared 4 platinum-based regimens as adjuvants found no significant difference between them in terms of overall survival or disease-free survival in patients with non-small cell lung cancer (NSCLC) (Figure 1).
In follow up that ranged from 40.6 months for pemetrexed to 60.3 months for docetaxel, investigators found no significant difference in overall survival or disease free survival based on chemotherapy regimen used. Adverse effects varied, with vinorelbine patients having more neutropenia and gemcitabine patients having more thrombocytopenia. Regimen made no difference in grade 3-5 adverse effects in squamous patients, but non-squamous patients who received pemetrexed had significantly less grade 3-5 toxicity than those that received other adjuvants.
with a massive host inflammatory response.
(Sources: Wikimedia/Wikimedia Commons/By Yale Rosen/Flikr.)
The E1505 study initially randomly assigned 1501 patients with early-stage, resected NSCLC to received either treatment consisting of chemotherapy alone or chemotherapy with bevacizumab. Early findings from that study showed no difference in overall survival between the two arms. NSCLC comprises a subset of all lung cancers (Figure 2.)
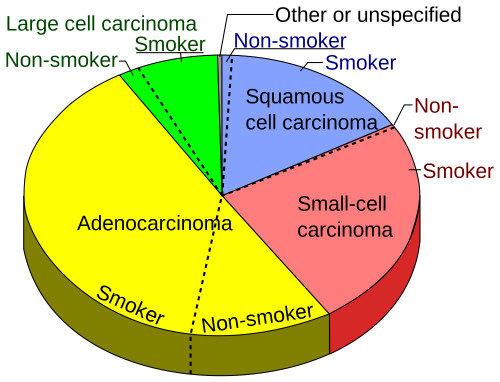
(Sources: Wikimedia/Creative Commons/By Mikael Häggström/Public domain.)
The results were presented at the 2016 annual meeting of the American Society of Clinical Oncology (ASCO) and are awaiting publication in a peer-reviewed journal. The trial was presented as abstract 8507: “E1505: Adjuvant chemotherapy ± bevacizumab for early stage NSCLC—Outcomes based on chemotherapy subsets.”
The research was overseen by Heather A. Wakelee, MD, Associate Professor of Medicine (oncology) at the Stanford University Medical Center in Palo Alto, California. Dr. Wakelee is the faculty director of the Stanford Cancer Clinical Trials office and is the lead investigator for ECOG-ACRIN clinical trials group at Stanford.

According to the ASCO presentation, the investigators analyzed the effects of adjuvant chemotherapy. Because bevacizumab had not affected outcomes, patients in both original arms were pooled for this analysis.
The chemotherapy regimen was 4 cycles of every-3-week cisplatin plus 1 of 4 adjuvant therapies: vinorelbine, docetaxel, gemcitabine or pemetrexed (for patients with non-squamous disease only). Adjuvant therapy had been investigator’s choice, so patients were not randomly assigned. Instead, 25% received vinorelbine, 23% docetaxel, 19% gemcitabine, and 33% pemetrexed. The arms were well balanced for known prognostic factors; 28% of all patients had squamous histology.
